Classification of matter pogil key – Embark on a journey through the fascinating world of matter classification with our comprehensive POGIL key. Discover the intricacies of matter’s composition, properties, and behavior, unlocking a deeper understanding of the physical world around us.
Delve into the diverse states of matter, unravel the mysteries of chemical properties, and explore the significance of chemical composition. Our guide will equip you with the knowledge to differentiate between mixtures and pure substances, witness the transformative power of phase changes, and grasp the practical applications of matter classification in various fields.
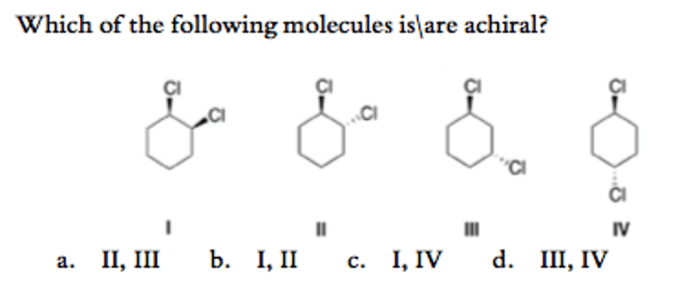
Classification of Matter: Classification Of Matter Pogil Key
Matter, the physical material of the universe, exhibits distinct properties that allow us to classify it into various categories. This classification system helps us understand the composition and behavior of matter in different forms.Matter exists in three primary states: solid, liquid, and gas.
Each state possesses unique characteristics that distinguish it from the others. Understanding these states is crucial for comprehending the behavior of matter in various contexts.
States of Matter, Classification of matter pogil key
- Solids: Solids possess a definite shape and volume. Their particles are tightly packed, resulting in a rigid structure. Examples of solids include ice, metal, and wood.
- Liquids: Liquids have a definite volume but no definite shape. Their particles are loosely packed, allowing them to flow and take the shape of their container. Examples of liquids include water, oil, and milk.
- Gases: Gases have no definite shape or volume. Their particles are widely dispersed and move freely. Examples of gases include air, helium, and hydrogen.
Physical and Chemical Properties
Physical and chemical properties are characteristics that describe matter. Physical properties are those that can be observed or measured without changing the composition of the matter. Chemical properties describe how matter changes when it reacts with other substances.
Physical Properties
- Color
- Odor
- Density
- Melting point
- Boiling point
- Solubility
- Electrical conductivity
- Thermal conductivity
Chemical Properties
- Flammability
- Reactivity with acids
- Reactivity with bases
- Reactivity with metals
- Reactivity with nonmetals
- Oxidation
- Reduction
Physical and chemical properties can be used to classify matter. For example, metals are typically shiny, malleable, and good conductors of heat and electricity. Nonmetals are typically dull, brittle, and poor conductors of heat and electricity. Acids are typically sour, corrosive, and react with bases to form salts.
Bases are typically bitter, slippery, and react with acids to form salts.
Chemical Composition
Chemical composition refers to the specific elements and the proportions in which they are present in a substance. It determines the chemical properties and behavior of the substance.
Chemical compounds are substances formed when atoms of different elements combine chemically in fixed proportions. There are various types of chemical compounds, including:
Ionic Compounds
- Formed by the transfer of electrons from one atom to another.
- Typically consist of a metal and a nonmetal.
- Example: Sodium chloride (NaCl) is an ionic compound formed from sodium (Na) and chlorine (Cl).
Covalent Compounds
- Formed by the sharing of electrons between atoms.
- Typically consist of nonmetals.
- Example: Water (H2O) is a covalent compound formed from hydrogen (H) and oxygen (O).
Molecular Compounds
- A type of covalent compound that exists as discrete molecules.
- Formed when two or more nonmetal atoms share electrons.
- Example: Carbon dioxide (CO2) is a molecular compound formed from carbon (C) and oxygen (O).
Metallic Compounds
- Formed by the bonding of metal atoms.
- Have a metallic luster and are good conductors of heat and electricity.
- Example: Iron (Fe) is a metallic compound that forms various alloys, such as steel.
Mixtures and Pure Substances
Mixtures are combinations of two or more chemical substances that are not chemically bonded. The substances retain their identity and are mixed in different forms, for example, solutions, suspensions, or colloids. Pure substances, on the other hand, consist of only one type of atom or molecule and have a fixed composition.
Types of Mixtures
Mixtures can be classified into three main types:
- Homogeneous mixtures: These mixtures have a uniform composition throughout. Examples include saltwater and air.
- Heterogeneous mixtures: These mixtures have a non-uniform composition, and their components can be distinguished physically. Examples include sand in water and oil in water.
- Colloids: Colloids are mixtures where one substance is dispersed in another in the form of very fine particles. Examples include milk and fog.
Separating Mixtures and Pure Substances
Mixtures and pure substances can be separated using various methods, depending on their physical and chemical properties. Some common methods include:
- Filtration: This method is used to separate solids from liquids. A filter paper is used to trap the solid particles, allowing the liquid to pass through.
- Distillation: This method is used to separate liquids with different boiling points. The mixture is heated, and the components with lower boiling points vaporize and are collected.
- Chromatography: This method is used to separate mixtures based on the different rates at which their components move through a stationary phase.
Phase Changes
Phase changes are physical processes in which a substance changes from one phase to another, such as from solid to liquid, liquid to gas, or vice versa. These changes are driven by changes in temperature and pressure.
Types of Phase Changes
The three main types of phase changes are:
- Melting: The change from a solid to a liquid phase, caused by an increase in temperature or a decrease in pressure.
- Freezing: The change from a liquid to a solid phase, caused by a decrease in temperature or an increase in pressure.
- Vaporization: The change from a liquid to a gas phase, caused by an increase in temperature or a decrease in pressure.
- Condensation: The change from a gas to a liquid phase, caused by a decrease in temperature or an increase in pressure.
- Sublimation: The change from a solid directly to a gas phase, bypassing the liquid phase, caused by an increase in temperature and a decrease in pressure.
- Deposition: The change from a gas directly to a solid phase, bypassing the liquid phase, caused by a decrease in temperature and an increase in pressure.
Applications of Phase Changes
Phase changes have numerous applications in everyday life and industry, including:
- Cooling and refrigeration: Vaporization and condensation are used in refrigerators and air conditioners to remove heat from a space.
- Power generation: Steam turbines use the expansion of vaporized water to generate electricity.
- Food preservation: Freezing and canning prevent food spoilage by inhibiting microbial growth.
- Material processing: Phase changes are used in casting, welding, and other manufacturing processes to shape and join materials.
- Chemical reactions: Phase changes can affect the rates and products of chemical reactions.
Understanding phase changes is essential for comprehending the behavior of matter and its applications in various fields.
Real-World Applications
Matter classification plays a pivotal role in numerous fields, driving technological advancements and shaping our understanding of the world around us. It finds practical applications in industries, research, and everyday life.
In industries, matter classification is crucial for material selection and product development. Engineers and scientists utilize knowledge of matter properties to design materials with specific characteristics, such as strength, durability, and electrical conductivity. This enables the creation of advanced materials for various applications, including construction, transportation, and electronics.
Research
In research, matter classification aids in the identification and characterization of new materials. Scientists use analytical techniques to determine the composition and properties of substances, which helps in understanding their behavior and potential applications. This knowledge contributes to the development of new technologies, such as energy storage systems and medical treatments.
Everyday Life
In everyday life, matter classification has practical implications. For instance, understanding the properties of different materials helps us make informed decisions about products we use, such as clothing, food, and cleaning supplies. Additionally, knowledge of chemical reactivity and toxicity enables us to handle and store chemicals safely, minimizing risks to our health and the environment.
Top FAQs
What is the significance of understanding matter classification?
Matter classification provides a systematic framework for organizing and comprehending the vast array of substances in the universe. It enables us to predict their behavior, properties, and interactions, which is crucial for scientific research, technological advancements, and everyday life.
How can physical properties be used to classify matter?
Physical properties, such as density, solubility, and melting point, can be used to distinguish between different substances. By comparing these properties, scientists can categorize matter into solids, liquids, gases, and other states.
What is the difference between a mixture and a pure substance?
A mixture is a combination of two or more substances that retain their individual identities. A pure substance, on the other hand, is a substance that has a uniform composition and cannot be further broken down into simpler components.