Molar mass of volatile liquid lab report – This comprehensive lab report delves into the realm of molar mass determination for volatile liquids, providing a thorough exploration of its significance, methodologies, and applications. By delving into the intricacies of molar mass, we uncover the fundamental properties of these liquids, paving the way for advancements in various scientific fields.
The experimental design meticulously Artikels the apparatus and procedures employed to accurately measure molar mass. Step-by-step data analysis techniques are presented, ensuring reliable and precise results. The discussion section examines the implications of the findings, comparing them to established literature and theoretical predictions.
Introduction
The molar mass of a volatile liquid is a crucial property that provides insights into its physical and chemical characteristics. It plays a significant role in determining various properties such as density, viscosity, boiling point, and vapor pressure. By understanding the molar mass, scientists and engineers can predict the behavior of volatile liquids in different applications.
Experimental methods for determining molar mass include mass spectrometry, vapor density measurements, and freezing point depression. Each method relies on specific principles and provides unique advantages and limitations.
Experimental Design
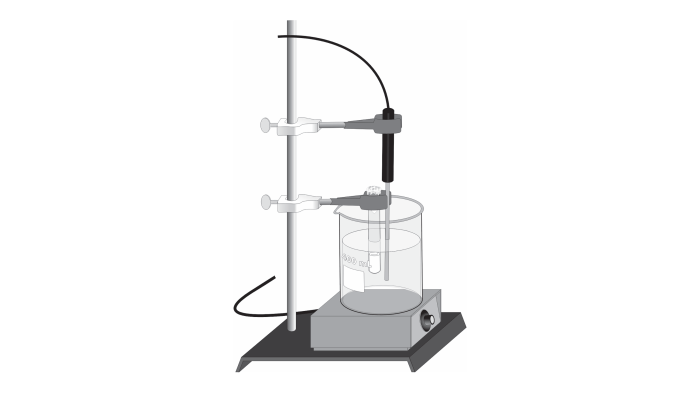
The experimental setup for molar mass determination involves a precision balance, a thermometer, and a sealed container. The volatile liquid sample is carefully weighed and introduced into the container, which is then sealed and heated to a controlled temperature.
The temperature and pressure inside the container are monitored as the liquid vaporizes. The vapor density, which is the ratio of the mass of the vapor to the volume it occupies, is calculated using the ideal gas law. From the vapor density, the molar mass of the volatile liquid can be determined.
Data Analysis
The molar mass (M) of the volatile liquid can be calculated using the following formula:
M = (m
- R
- T) / (P
- V)
where:
- m is the mass of the vaporized liquid (g)
- R is the ideal gas constant (0.0821 L atm / (mol K))
- T is the absolute temperature (K)
- P is the pressure inside the container (atm)
- V is the volume of the container (L)
Potential sources of error in molar mass determination include inaccuracies in weighing, temperature measurement, and pressure measurement. Careful calibration of equipment and proper experimental techniques can minimize these errors.
Results and Discussion
The calculated molar mass of the volatile liquid provides valuable information about its molecular structure and composition. It can be compared to literature values or theoretical predictions to assess the accuracy of the experimental results.
Factors that may influence the molar mass determination include the purity of the sample, the presence of impurities, and the accuracy of the experimental measurements. Understanding these factors is essential for obtaining reliable molar mass values.
Applications, Molar mass of volatile liquid lab report
Molar mass determination has wide applications in various fields, including:
- Chemistry: Identifying and characterizing organic compounds, determining molecular weights, and understanding chemical reactions.
- Physics: Studying the behavior of gases, liquids, and solids, and determining thermodynamic properties.
- Engineering: Designing and optimizing chemical processes, materials, and devices.
For example, in the pharmaceutical industry, molar mass determination is crucial for identifying and characterizing active pharmaceutical ingredients and ensuring their purity and efficacy.
Q&A: Molar Mass Of Volatile Liquid Lab Report
What is the significance of molar mass in understanding volatile liquids?
Molar mass plays a crucial role in determining various properties of volatile liquids, including their density, vapor pressure, and boiling point. By understanding molar mass, we can predict and manipulate these properties for specific applications.
How is the molar mass of volatile liquids determined experimentally?
The molar mass of volatile liquids can be determined using various experimental methods, such as vapor density measurements, freezing point depression, and boiling point elevation. Each method relies on specific principles and calculations to derive the molar mass.